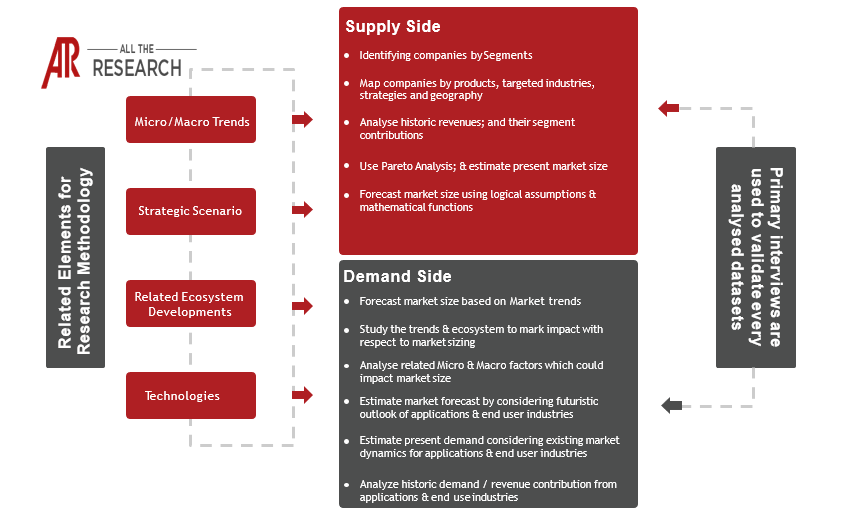
The Point-of-Care (POC) diagnostics market is a rapidly evolving segment of the healthcare industry, focused on providing rapid, accessible, and decentralized diagnostic testing solutions. This market encompasses devices, consumables, and software designed to deliver diagnostic results at or near the patient, allowing for faster diagnosis, treatment initiation, and improved patient outcomes. This report provides a comprehensive analysis of the POC diagnostics market, examining its current state, future growth prospects, and key influencing factors.
Market Scope and Definitions:
The POC diagnostics market is characterized by its broad application across various clinical settings, including physician's offices, pharmacies, emergency rooms, operating rooms, and even home settings. Key product segments within the market include:
Key Market Drivers:
The POC diagnostics market is experiencing significant growth, fueled by several key drivers:
Key Challenges:
Despite its growth potential, the POC diagnostics market faces certain challenges:
Regulatory Landscape and Major Players:
The regulatory landscape is highly focused on ensuring patient safety and test accuracy. Key regulatory bodies include the FDA (US), CE marking (Europe), and comparable agencies in other countries. The market is characterized by the presence of both large multinational corporations and smaller, specialized players. Key market players include: Abbott, Roche, Siemens Healthineers, Beckman Coulter, BD, QuidelOrtho, and others.
Regional Trends and Emerging Developments:
Trends in M&A and Fundraising:
The POC diagnostics market is witnessing significant activity in M&A and fundraising. Companies are engaging in strategic acquisitions to expand their product portfolios, geographic reach, and technological capabilities. Fundraising activities, including venture capital investments and public offerings, are supporting innovation and the development of new POC solutions. This trend is particularly evident in areas such as infectious disease diagnostics, molecular testing, and the integration of POC devices with digital health platforms.
CAGR and Future Outlook:
The global POC diagnostics market is projected to experience robust growth over the forecast period, with a CAGR (Compound Annual Growth Rate) that fluctuates between 7% and 10%, depending on the specific segment and regional dynamics. The market's future outlook is promising, with further advancements in technology, increasing demand for decentralized healthcare, and growing emphasis on improved patient outcomes.
The Report Segments the market to include:
Product Type
Application
End User
Region
Mode of Purchase

Ask for free product review call with the author

Share your specific research requirements for a customized report
Request for due diligence and consumer centric studies

Request for study updates, segment specific and country level reports
Product Type
Application
End User
Region
Mode of Purchase
MEDICA (Düsseldorf, Germany): (November 2024) World Forum for Medicine, showcasing POC diagnostics alongside broader medical technologies.
AACC Annual Scientific Meeting & Clinical Lab Expo (Various Locations, US): (July/August 2024 & 2025) Focused on laboratory medicine, including POC testing, with educational sessions, presentations, and an extensive exhibition.
POCT (Point of Care) Conference (Various Locations, Global): (Ongoing/upcoming) Specific events dedicated to POCT, discussing regulatory issues, technologies, and implementation strategies.
European Lab Automation (ELA) (Various Locations, Europe): (May 2025) Focuses on lab automation that can be relevant to POC, including aspects of decentralised diagnostics.
Diagnostics World (Various Locations, Global): (Ongoing/upcoming) Series of global conferences and webinars dedicated to the diagnostics industry, including POC.
CLMA (Clinical Laboratory Management Association) Conference (Various Locations, US): (Ongoing) Specifically addresses POC, focusing on effective lab management, including POCT.
RAPID + TCT (Various Locations, US): (Ongoing) Whilst focused on 3D printing/additive manufacturing, many applications for POCT are starting to be featured.
Webinars from Industry Organizations/Companies: Regular webinars from companies, industry associations (e.g., AACC, CLSI), and research institutions, providing up-to-date information on specific POC technologies and market trends.
SLAS (Society for Laboratory Automation and Screening) Conferences (Various Locations, Global): (Ongoing) Focused on lab automation and innovative technologies, some of which relate to POC.
Abbott Roche Siemens Healthineers Danaher BD (Becton, Dickinson and Company) Johnson & Johnson bioMérieux Sysmex QuidelOrtho Beckman Coulter (Danaher) EKF Diagnostics Nova Biomedical Hologic Sekisui Diagnostics Thermo Fisher Scientific Accriva Diagnostics Trinity Biotech PointCheck Convergent Technologies Response Biomedical